Voltage Imaging
Membrane voltage is an important biophysical signal that affects many fundamental aspects of cellular physiology. The transmembrane electric field perturbs the energy landscape of biomolecules embedded in the lipid membrane, including ion channels, G protein-coupled receptors and membrane-associated enzymes, and is affected in turn by the dynamics of voltage- and ligand-gated ion channels, as well as electrogenic transporters and pumps. While electrical signaling is most commonly associated with excitable cells such as neurons and cardiomyocytes, it is also observed in many other cell types (e.g. bacteria and plants) and organelles (e.g. mitochondria). Voltage imaging with fluorescent indicators has enabled direct visualization of the spatial pattern and temporal dynamics of electrical signaling at the cellular and circuit levels (Curr. Opin. Chem. Biol., 2017).
In the past, we have created a palette of fluorescent voltage-indicating proteins that span much of the visible spectrum. These sensors are based on a novel voltage-sensing mechanism called electrochromic FRET (eFRET). In eFRET, a fluorescent protein is the FRET donor and the retinal chromophore in a rhodopsin protein is the FRET acceptor. Membrane voltage shifts the absorption spectrum of the retinal chromophore, thereby modulating the FRET efficiency. By measuring the changes in fluorescent protein emission, we could detect membrane action potential spikes in neurons via fluorescence microscopy (Nat. Commun., 2014).
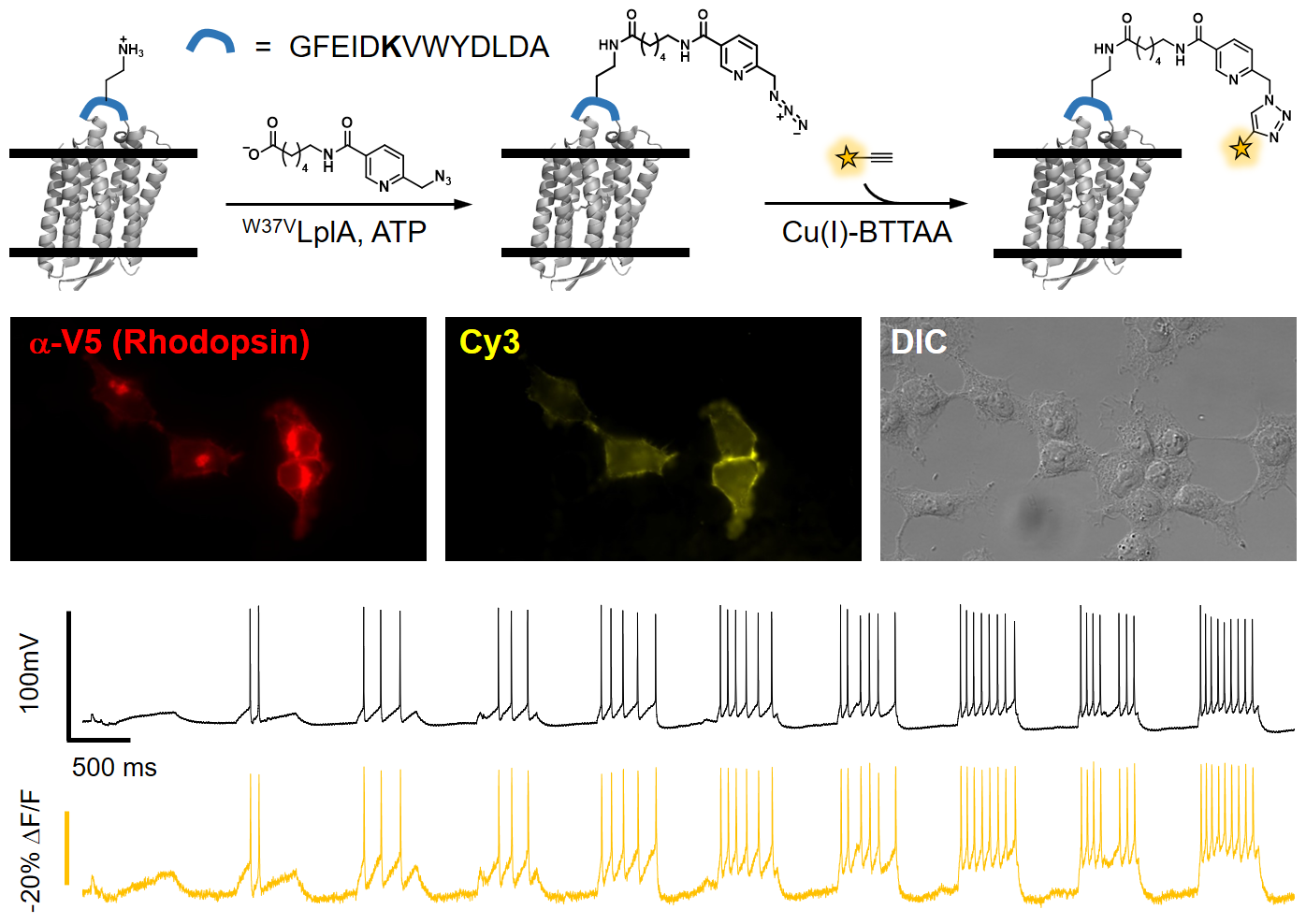
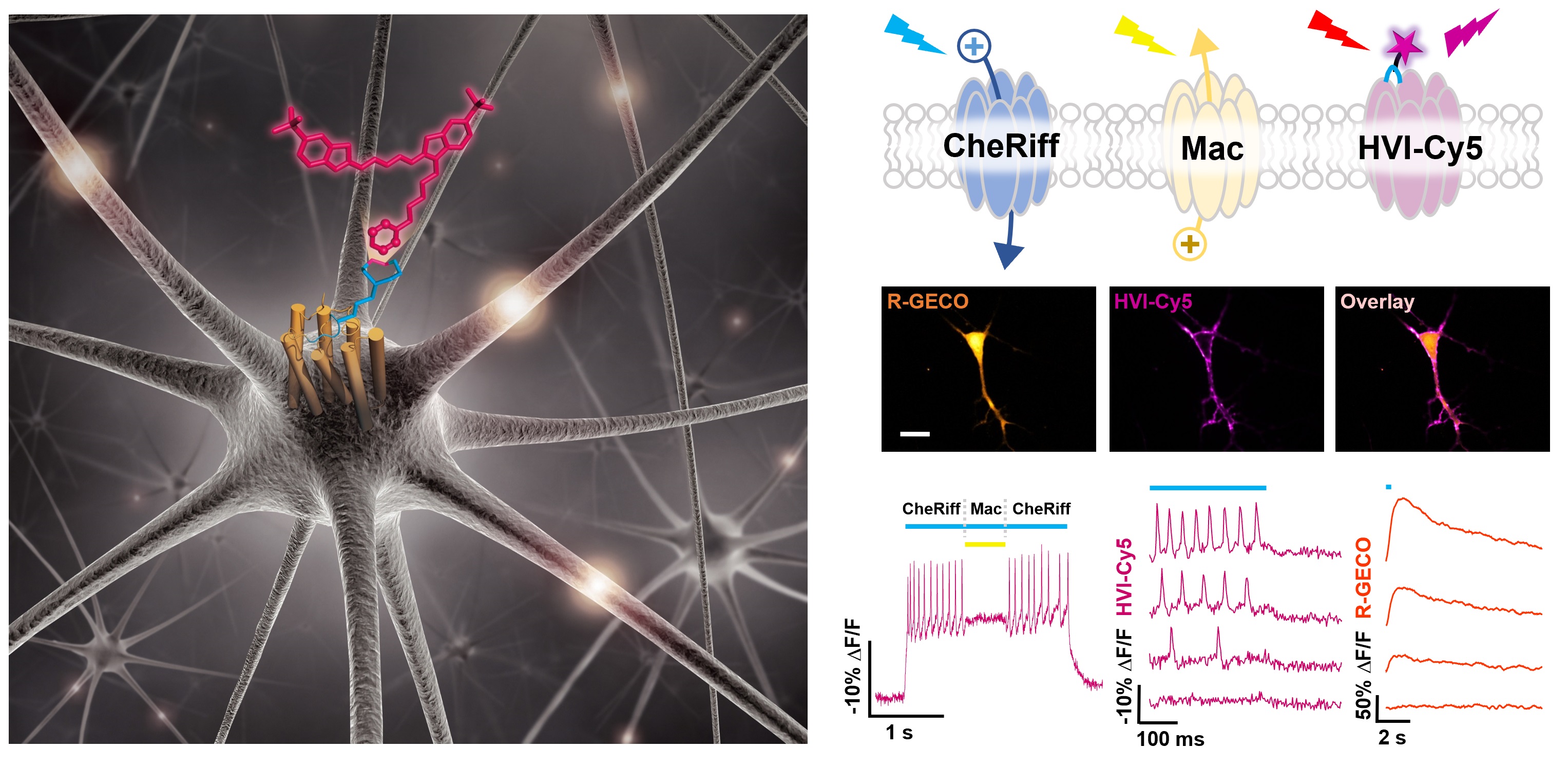
More recently, we have expanded the scope of eFRET voltage sensing by designing a small molecule/protein hybrid indicator (ACS Chem. Neurosci., 2018). This method, called Flare (fluorophore ligation-assisted rhodopsin eFRET), has submillisecond response kinetics. We applied various site-specific protein labeling techniques, including HaloTag, SNAP-tag, and enzyme-mediated probe incorporation, to ligate fluorophores to a rhodopsin protein. The most sensitive indicator of this series, Flare1, allows us to investigate the spatial and temporal correlation of electrical coupling among mammalian cells (Angew. Chem. Int. Ed. Engl., 2018). Further optimizations through both structure-guided mutagenesis (ACS Chem. Neurosci., 2019) and using inverse electron demand Diels-Alder reaction has led to the develoment of far-red hybrid voltage indicator, HVI-Cy5. The improved voltage sensitivity (-39% ΔF/F per 100 mV) and red-shifted emission spectrum of HVI-Cy5 allows for multiplexed imaging of membrane potential and other neuronal signals, such as calcium and neurotransmitter release. It also enables all-optical investigation of neuronal electrophysiology (Nat. Chem., 2021). Future developments are underway to achieve voltage imaging in vivo.